As of 2011, NPIC stopped creating technical pesticide fact sheets. The old collection of technical fact sheets will remain available in this archive, but they may contain out-of-date material. NPIC no longer has the capacity to consistently update them. To visit our general fact sheets, click here. For up-to-date technical fact sheets, please visit the Environmental Protection Agency's webpage.
Laboratory Testing: Before pesticides are registered by
the U.S. EPA, they must undergo laboratory testing for
short-term (acute) and long-term (chronic) health effects.
Laboratory animals are purposely given high enough doses
to cause toxic effects. These tests help scientists judge how
these chemicals might affect humans, domestic animals,
and wildlife in cases of overexposure.
Molecular Structure - Acephate
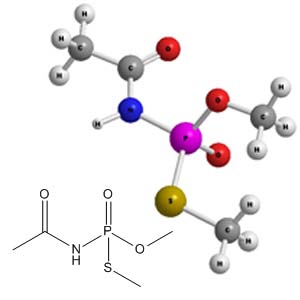
- Acephate is an organophosphate insecticide.1 The
International Union of Pure and Applied Chemistry (IUPAC) chemical
name for acephate is O,S-Dimethyl acetylphosphoramidothioate2, and the
Chemical Abstracts Service (CAS) registry number is
30560-19-1.1
- Acephate was first registered for use by the United States Environmental
Protection Agency (U.S. EPA) in 1973. Reregistration
for acephate was granted in July 2006.1 See the text box on Laboratory
Testing.
- In soil, plants, and insects, acephate is converted to methamidophos.
Methamidophos is another organophosphate insecticide
that is registered by the U.S. EPA.1
- Technical grade acephate is a white or transparent solid.1 Acephate has a
strong odor similar to mercaptan, which smells like sulfur.3
- Vapor pressure1: 1.7 x 10-6 mmHg at 24 °C
- Octanol-Water Partition Coefficient (Kow)4: 0.13 at 25 °C
- Henry's constant5,6: 3.1 x 10-7 atm·m3/mol; 5.1 x 10-13 atm mole/m3
- Molecular weight1: 183.16 g/mol
- Solubility (water)1,6: 79 - 83.5 g/100 mL
- Soil Sorption Coefficient (Koc)2,6: 2; 2.7
- Acephate is a general-use insecticide registered for use on food crops, agricultural
seed and non-bearing plants, institutions and commercial buildings
including public health facilities, sod, golf course turf, ant mounds, and horticultural
nursery plants.1
- Signal words for products containing acephate may range from Caution to Danger. The signal word reflects the combined
toxicity of the active ingredient and other ingredients in the product. See the pesticide label on the product and refer to
the NPIC fact sheets on Signal Words and Inert or "Other" Ingredients.
- To find a list of products containing acephate which are registered in your state, visit the website
https://npic.orst.edu/reg/state_agencies.html select your state then click on the link for "State Products."
Target Organisms
- Acephate is a systemic insecticide used to control sucking and biting insects by direct contact or ingestion.7,8
- Organophosphates such as acephate bind to and inhibit the enzyme acetylcholinesterase (AChE) in nervous system tissues.
As a result, the neurotransmitter acetylcholine accumulates and repeatedly activates cholinergic receptors.9,10 Acephate itself
is a weak acetylcholinesterase inhibitor.11 Methamidophos is a more potent organophosphate than acephate.10 Insects
metabolize acephate into methamidophos by hydrolysis, whereas mammals metabolize acephate more readily into des-
O-methylacephate, accounting for acephate's relatively high selectivity against insects.2,12,13
- Methamidophos inhibits acetylcholinesterase through phosphorylation.12 Acetylcholine is the prominent insect stimulatory
neurotransmitter for motor, sensory, and intermediate neurons,14 and is broken down by acetylcholinesterase.15 Organophosphates
cause acetylcholine levels to increase and over-excite target nerves, muscles or tissues.10
Non-target Organisms
- Mammals are less sensitive to acephate than insects.2,11 The selectivity or potency of acephate is tied to mammalian metabolism.13
Mammalian metabolism of acephate into the cholinesterase inhibitor methamidophos is minor, compared to
the main pathway of its conversion into des-O-methylacephate, also known as S-methylacetyl phosphoramidothiolate
(SMPT), which is not a cholinesterase inhibitor.2,11
- Organophosphates such as acephate and methamidophos inhibit cholinesterase in mammals.10 However, acephate is
a weak cholinesterase inhibitor.10,11 Methamidophos is more toxic to invertebrates and vertebrates alike, compared to
acephate.6
- Other studies and authors have suggested that acephate blocks methamidophos from binding to acetylcholine directly or
by an allosteric site in mammals.16 See the section on Metabolism.
- In rat studies in vitro, methamidophos inhibited erythrocyte and brain acetylcholinesterase a thousand times more effectively
than acephate.17 Another study indicated methamidophos is ten times stronger at inhibiting cholinesterase than
acephate in mice.11
- One study on white throated sparrow indicated brain cholinesterase inhibition after ingestion of acephate.18 A study in
which hens were given acephate by gavage showed inhibition of acetylcholinesterase and, to a much lesser extent, neuropathy
target esterase.19
- Acephate is generally considered non-phytotoxic, but leaf burn on Red Delicious apples after acephate application has
been observed.7
LD50/LC50: A common
measure of acute toxicity is the lethal dose (LD50) or
lethal concentration (LC50) that causes death (resulting
from a single or limited exposure) in 50 percent of the treated
animals. LD50 is generally expressed as the dose in
milligrams (mg) of chemical per kilogram (kg) of body
weight. LC50 is often expressed as mg of chemical per
volume (e.g., liter (L)) of medium (i.e., air or water) the organism
is exposed to. Chemicals are considered highly toxic when the
LD50/LC50 is small and practically non-toxic
when the value is large. However, the LD50/LC50
does not reflect any effects from long-term exposure (i.e., cancer,
birth defects or reproductive toxicity) that may occur at levels below
those that cause death.
- Acephate breaks down to methamidophos in the environment
by microbes in the presence of oxygen.4 Methamidophos is
more toxic to birds and mammals than its parent compound,
acephate.1
Oral
- The oral LD50 for acephate is 1.4 g/kg in male rats, and 1.0 g/kg
in female rats.1 Acephate is low in toxicity when eaten. See the
text boxes on Toxicity Classification and LD50/LC50.
- The lowest lethal dose observed in a study of Beagle dogs was 680 mg/kg.20
- Human volunteers ingested a single dose of acephate. No physiological changes were observed at the highest doses
tested, 1.2 mg/kg in men and 1.0 mg/kg in women.20 See the text box on NOAEL, NOEL, LOAEL, and LOEL.
| TOXICITY CLASSIFICATION - ACEPHATE |
|
High Toxicity |
Moderate Toxicity |
Low Toxicity |
Very Low Toxicity |
| Acute Oral LD50 |
Up to and including 50 mg/kg
(≤ 50 mg/kg) |
Greater than 50 through 500 mg/kg
(>50-500 mg/kg) |
Greater than 500 through 5000 mg/kg
(>500-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Inhalation LC50 |
Up to and including 0.05 mg/L
(≤0.05 mg/L) |
Greater than 0.05 through 0.5 mg/L
(>0.05-0.5 mg/L) |
Greater than 0.5 through 2.0 mg/L
(>0.5-2.0 mg/L) |
Greater than 2.0 mg/L
(>2.0 mg/L) |
| Dermal LD50 |
Up to and including 200 mg/kg
(≤200 mg/kg) |
Greater than 200 through 2000 mg/kg
(>200-2000 mg/kg) |
Greater than 2000 through 5000 mg/kg
(>2000-5000 mg/kg) |
Greater than 5000 mg/kg
(>5000 mg/kg) |
| Primary Eye Irritation |
Corrosive (irreversible destruction of
ocular tissue) or corneal involvement or
irritation persisting for more than 21 days |
Corneal involvement or other
eye irritation clearing in 8 -
21 days |
Corneal involvement or other
eye irritation clearing in 7
days or less |
Minimal effects clearing in less than 24 hours |
| Primary Skin Irritation |
Corrosive (tissue destruction into the
dermis and/or scarring) |
Severe irritation at 72 hours
(severe erythema or edema) |
Moderate irritation at 72
hours (moderate erythema) |
Mild or slight irritation at
72 hours (no irritation or
erythema) |
| The highlighted boxes reflect the values in the "Acute Toxicity" section of this fact sheet. Modeled after the U.S. Environmental Protection Agency, Office of Pesticide Programs, Label Review Manual, Chapter 7: Precautionary Labeling. https://www.epa.gov/sites/default/files/2018-04/documents/chap-07-mar-2018.pdf |
Dermal
NOAEL: No Observable Adverse Effect Level
NOEL: No Observed Effect Level
LOAEL: Lowest Observable Adverse Effect Level
LOEL: Lowest Observed Effect Level
- The dermal LD50 was greater than 10,000 mg/kg in male rabbits.1
- Acephate is not a skin sensitizer in guinea pigs.1,17,21
- Acephate is not considered an eye irritant by the EPA, based on a
study in rabbits.1
- In another study, acephate caused inflammation of the eye membrane and iris, and minor corneal opacity in rabbits. Symptoms
were reversed within two weeks.17,22
- After administering up to 0.1 mL of acephate, slight eye redness and fluid discharge were observed in rabbits. When
eyes were rinsed a minute after treatment, researchers noticed swelling. In both cases, rabbits' eyes recovered within 24
hours.17,23
Inhalation
- Acephate is very low in toxicity when inhaled, with an LC50 of greater than 61.7 mg/L.1
- The LC50 value for inhalation of aerosol containing acephate dissolved in distilled water is greater than 15 mg/L air over 4
hours in rats.17,24
- Rats were exposed exclusively to air passed over acephate crystals for four hours. No fatalities or changes in cholinesterase
levels were observed.25
- Rats were exposed to aerosol droplets containing from 0-4% acephate for an hour every day, five days a week for two
weeks. No effects were observed at 1% acephate. Increases in uric acid were observed in females after exposure to 4%
acephate. No other clinical changes were observed.25
Signs of Toxicity - Animals
- Acephate ingestion in mice caused muscular weakness, tremors, and reduced activity. Signs resolved within nine days in
survivors.20
- Acephate ingestion in rats caused depression, decreased food consumption, lethargy, diarrhea, excess salivation, urinary
incontinence, tremors, ataxia, and collapse. Signs in survivors resolved within eight days of administration.20
- Acephate ingestion in beagle dogs resulted in muscular tremors, diarrhea, emesis, dyspnea, ataxia, clonic convulsions and
bloody diarrhea until six hours after acephate administration.20
- Dermal exposure to acephate on rabbits caused tremors, diarrhea and dermal redness and inflammation of the treated
skin.20
- Acephate inhalation in rats caused excess salivation, tremors, ataxia and lethargy. Signs resolved within four days of administration.20
- Signs of toxicity noted in birds were incoordination, depression, shortness of breath, feather puffing, dropped wings, falling
rigidly with outspread wings, lying down, tremors, and convulsions. Signs and death have been observed as soon as 30
minutes and five hours, respectively, after acute ingestion.26
Signs of Toxicity - Humans
- Symptoms appear rapidly if the organophosphates are inhaled, somewhat slower if ingested, and more delayed following
dermal exposure. Symptoms from organophosphates can become apparent within minutes to hours after exposure,
depending on the exposure route.10
- Symptoms include headache, nausea, dizziness and confusion.1,10 Incident reports to the EPA about exposure to acephate containing
pesticides were most frequently associated with gastrointestinal, neurological, respiratory, or dermal symptoms
such as nausea, diarrhea, abdominal cramps, tremors, tachycardia, sweating, disorientation, coughing, wheezing, congestion,
and pneumonia. Skin irritation and reactions such as swelling, hives, redness, and rashes have also been reported.27
- Overexcitation of central nervous system from organophosphates may result in moodiness or agitation, confusion, lethargy,
weakness, convulsions, incoordination, memory loss, cyanosis or coma.9 When the muscarinic receptors are over-excited in
the parasympathetic nervous system, cholinergic signs and symptoms including abdominal cramps and diarrhea, hypersecretion,
urination, tightening of the bronchi, decreased or increased heart rate, and miosis may occur.9 Severe exposures
to acephate can cause respiratory paralysis and death.1
- Children can experience different symptoms than adults if exposed to organophosphates, including seizures, lethargy, and
coma, flaccid muscle weakness, miosis and excessive salivation.10
- Always follow label instructions and take steps to minimize exposure. If any exposure occurs, be sure to follow the First Aid
instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 800-222-1222. If you wish to discuss an incident with the National Pesticide Information Center, please call 800-858-7378.
Animals
- Acephate was given to rats in their diets at concentrations of 0, 2, 5, 10, and 150 ppm for 13 weeks. After 13 weeks, cholinesterase
activity in the brain and red blood cells was inhibited at all doses. The red blood cell and plasma cholinesterase inhibition
NOEL was 0.58 mg/kg/day and 0.76 mg/kg/day for male and female rats, respectively. Brain cholinesterase inhibition
was detected in all treated rats. No inhibition of plasma or red blood cell cholinesterase was observed at 10 ppm doses.28
- Rats were given 1 or 10 mg/kg acephate by gavage daily for 15 weeks.29 Brain acetylcholinesterase activity changes were not
observed in either group.29 Decreases in plasma and red blood cell cholinesterase activities, and increased catecholamine
were observed in the 10 mg/kg treatment group.29 Brains from acephate-treated rats showed increased epinephrine, norepinephrine,
glutamine and asparagine and decreased GABA, dopamine, glutamic acid decarboxylase and tyrosine compared
to controls.29 Rats in the higher treatment group had increased urination, fasciculation, and hyperactivity in the last
few weeks of treatment.29
- Brain cholinesterase inhibition was observed in both sexes of beagle dogs given 120 or 800 ppm per day acephate for 52
weeks. In males, the effect was also seen at 10 ppm. No overt symptoms were observed in the dogs.20
- In a study of oral acephate exposure in hens, authors concluded that organophosphate-induced delayed neuropathy
(OPIDN) was implausible based on the low neuropathy target esterase inhibition at acephate levels that were life threatening
or fatal to tested hens.19 In another study, hens were administered the median lethal oral dose of acephate in a delayed
neurotoxicity study. No evidence of delayed neurotoxicity such as neurotoxic symptoms or neural tissue damage was
observed.17
Humans
- Fourteen volunteers ingested mixtures of acephate and methamidophos. Plasma cholinesterase inhibition was observed,
and the LOAEL was established for a 1:4 ratio of methamidophos:acephate at 0.2 mg/kg/day in both sexes after 16 days of
administration.25 No other changes were observed. Cholinesterase activities returned to normal within seven days.17 See
the text box on Exposure.
Exposure: Effects of acephate on human health and the environment depend on how much
acephate is present and the length and frequency of exposure. Effects also depend on the health
of a person and/or certain environmental factors.
- Ten male volunteers each ingested 0.25 mg/kg/day acephate for 28 days. No acetylcholinesterase inhibition, clinical or
physiological changes were observed.20
- The intermediate syndrome has been attributed to methamidophos following acute, symptomatic poisoning. Health effects
from intermediate syndrome may include limb, neck, and respiratory muscle weakness, or death. Intermediate syndrome
onset begins a day or more after initial acute symptoms begin or resolve and may last 30 to 40 days. No cases linking
these conditions to acephate were found.
- Organophosphate-induced delayed (poly)neuropathy (OPIDN) has been reported from methamidophos exposure. OPIDN
onset is two or three weeks after some acute poisonings, and has only been observed in cases with acute cholinergic
symptoms. OPIDN effects may include leg muscle pain, followed by numbness, weakness or paresthesia beginning in the
feet and sometimes hands.30
- In one study, reversible hyperglycemia and adrenal cortex hyperactivity were observed two to six hours after fasted male
rats were fed 140 mg/kg acephate.31
- In another study, rats were given 178 mg/kg acephate by gavage daily for four, fourteen, or sixty days.32 Overall, brain
serotonin and metabolite levels decreased after treatment, but the pattern of change varied in different sections of the
brain.32
- Female Wistar rats were injected with 500 mg/kg acephate or 5 mg/kg methamidophos intra-peritoneally. Increases in
serum corticosterone and aldosterone, and decreases in serum glutamate and histidine levels were observed after both
treatments. According to the authors, the results suggest that acephate and methamidophos injection increase energy
needs and gluconeogenesis in treated rats.16
- Acephate or methamidophos exposure to rat hypothalamus tissue isolated in vitro resulted in increased corticotropinreleasing
factor (CRF) expression and release, even in the presence of a factor that normally inhibits CRF.33
- Acephate is on U.S. EPA's June 2007 Draft List of Chemicals for Initial Tier 1 Screening list for the Endocrine Disruptor
Screening Program (EDSP).34 Priority for endocrine disruption testing is set for chemicals based on the likelihood of human
exposure to them.34
Animals
- Significant increases in hepatocellular carcinomas and adenomas were observed in female mice fed 1000 ppm acephate
in their diet for two years. In male rats that were fed the same doses, no significant increases in tumor incidence was noticed.17
- Only male rats showed increased frequency of adrenal gland tumors at dietary doses of acephate up to 700 ppm for 28
months. The relationship between tumor occurrence and acephate dose was not linear.17
- According to one source, acephate was a weak mutagen in studies of Salmonella, Escherichia coli, and Saccharomyces cerevisiae.28
Another source cited inconsistent genotoxicity results in acephate studies among several prokaryotic and eukaryotic
cell types.35
- Results from acephate genotoxicity studies using whole animals have been mixed, indicating both positive and negative
results.17,36
- In another study, male mice were fed acephate doses ranging from 12.25 to 392 mg/kg by intubation. DNA damage was
observed in cells of all treated mice. DNA damage repair began in lower dose groups by 72 hours and was complete in all
dose groups by 96 hours. The level of DNA damage correlated to the dose.35
Humans
- The U.S. EPA designated acephate as Group C, possible human carcinogen based on increases in liver adenomas and carcinomas
in female mice that ate acephate.28 See the text box on Cancer.
Cancer: Government agencies in the United States and abroad have developed programs to evaluate the
potential for a chemical to cause cancer. Testing guidelines and classification systems vary. To learn more
about the meaning of various cancer classification descriptors listed in this fact sheet, please visit the
appropriate reference, or call NPIC.
- No human data were found on carcinogenic effects of acephate.28
- Methamidophos is not likely to be a human carcinogen according to U.S. EPA classification.37
Animals
- In multi-generational and prenatal development studies, fetuses and pups were no more sensitive to acephate than the
adult rats or rabbits.1
- Male mice were fed acephate five days a week for four weeks at doses up to 28 mg/kg/day, and then mated with untreated
female mice.12 At doses over 7 mg/kg/day, they observed decreases in mating, male fertility, sperm counts and motility,
implantations and live fetuses, and increased resorptions.12 Testes weighed less in all treated mice.12
- Multigenerational exposure to acephate in rats resulted in reduced body weight gain, increased food consumption, and
reductions in mating and litter size.17
- No changes in nervous system development were found in multigenerational acephate exposure studies, including behavioral
and cognitive effects.1,20 No developmental or neurotoxic effects were observed in offspring at doses up to 10 mg/
kg/day.20
- In considering environmental risks, the U.S. EPA concluded that chronic exposure to acephate and methamidophos may
decrease offspring survival and body weight in wild mammals.1
Humans
- No human data were found on the teratogenic or reproductive effects of acephate.
Absorption
- When acephate is eaten, it is quickly absorbed from the gastrointestinal tract in rats and humans.20,38
- Acephate is readily absorbed by the skin of rats.17,39,40
- Acephate in aerosol formulations is absorbed by inhalation.17
Distribution
- In rats, dermally absorbed acephate is distributed into the blood. Blood concentrations of acephate were highest 1 to 3
hours after exposure.39,40
- In rats given acephate by oral gavage, acephate evenly distributes to tissues according to their level of blood circulation.
Accordingly, highest acephate concentrations were found in the skin, liver, kidney and heart, and were comparable to
blood plasma acephate concentrations.20
- In two human deaths involving presumed intentional ingestion, acephate was detected most in the stomach and urine,
and in lesser amounts in blood. One of the subjects had ingested 100 mL or less of a product containing 17.25% acephate.
The ratios of methamidophos to acephate in the stomach, urine and blood were at or below 2:100.41
Metabolism
- Acephate in mammals remains mostly unmetabolized.20 Mammalian metabolism of acephate into the more potent cholinesterase
inhibitor methamidophos is minor, compared to the main pathway of its conversion into the metabolite des-
O-methylacephate. The des-O-methylacephate is further metabolized into molecules that are integrated into cellular biochemicals,
or excreted.2
- After dermal exposure in rats, one study found 1% of administered acephate was excreted from urine as methamidophos.17,39
- In one study, urinary metabolites of acephate in rats after oral administration included 73-77% acephate, 3-6% O-S-dimethylphosphorothioate,
and 3-4% S-methyl-acetylphosphoramidothioate. Their assay did not indicate the presence of
methamidophos.40,42
- According to another study, microbes biodegraded a small percentage of the orally-given acephate to methamidophos in
the rat gastrointestinal tract; 0.5-1.5% of dosed acephate was detected both in rat tissues and in rat urine as methamidophos.17
- In one study, mice fed a single 120 μg/g dose of acephate by tube were evaluated for acephate metabolites in the liver.11
After thirty minutes, 19-30% acephate was detected in the liver. The major metabolite was S-methylacetyl phosphoramidothiolate,
which peaked at 8-9% seven hours after feeding. After 30 minutes, 1.5% of the dose was identified as methamidophos.
After 24-30 hours, only O, S dimethyl phosphorothiolate was detectable in the liver at very small levels.11
- In hens given acephate by gavage, the proportion of methamidophos detected in the brain decreased as doses increased.
At 25 mg/kg acephate doses, 16% methamidophos was observed; at 700 mg/kg acephate doses, methamidophos levels
averaged 10%.19
Excretion
- Acephate is rapidly excreted from mammals.25 After ingestion in rats, 82-95% of the acephate dose was rapidly excreted in
the urine, 1-9% through exhalation as carbon dioxide, and 1-2% in feces.40,42 Less than 1% of orally administered acephate
in rats was detected in tissues 72 hours after exposure.20,38
- Acephate plasma elimination was non-linear in rats; the elimination half-life is 1.4 hours during the first two hours, and 50
hours beginning 24 hours after the exposure.20
- After maternal oral exposures, rat offspring can be exposed to acephate through the placenta or milk.17
- One study measured urine levels of acephate and methamidophos in humans exposed to acephate while farming in fields.
Acephate was detected in urine, but methamidophos was not. Daily maximum acephate urine concentrations ranged from
3 to 9 mg/L.40 Urinary concentrations of acephate were undetectable in less than 48 hours after exposure ended.40
- Red blood cell (RBC) acetylcholinesterase or plasma butylcholinesterase inhibition can be measured as a biomarker of
effect for acute and chronic exposure to organophosphates.10,30,43 The level of RBC cholinesterase inhibition detected is
associated with symptom severity in poisonings.30
- Some agencies recommend or require periodic cholinesterase level testing for people who handle organophosphates in
their work.43,44,45 Blood cholinesterase levels differ widely among people, and in individuals over time.30,43 Workers are often
tested before any potential exposure to establish a baseline.43,44,45 Post-exposure blood cholinesterase samples can then be
compared to pre-exposure samples from the same individual.43
- When occupational exposures occur or a worker complains of illness, tests are optimally performed within 24 hours.43 Cholinesterases
are continuously resynthesized by the body.30
- Other methods of testing for organophosphate exposure exist, such as detecting organophosphate metabolites in urine.
However, they require advanced technology and are not usually available in clinical settings. Their clinical significance is
unclear.30,43
The "half-life" is the time required for half of the
compound to break down in the environment.
1 half-life = 50% remaining
2 half-lives = 25% remaining
3 half-lives = 12% remaining
4 half-lives = 6% remaining
5 half-lives = 3% remaining
Half-lives can vary widely based on environmental
factors. The amount of chemical remaining after a
half-life will always depend on the amount of the
chemical originally applied. It should be noted that
some chemicals may degrade into compounds of
toxicological significance.
Soil
- In soil, acephate breaks down primarily by aerobic metabolism and to a lesser degree by photolysis to methamidophos,
which is another organophosphate insecticide registered by the U.S. EPA.1,6 Twenty-three percent of applied acephate converted
to methamidophos by aerobic metabolism in loam soil, and less than 10% did so in two other soil types.6
- By soil photolysis, 8.4% or less converted to methamidophos.6 Methamidophos is then mineralized to carbon dioxide and
microbial biomass by soil microorganisms with a half-life of less than ten days.1,6 See the text box on Half-life.
- In laboratory studies, the calculated half-life of acephate ranged
from about 4.5 days in half-saturated silt loam soil to 32 days in halfsaturated
silt clay loam soil.5 The half-life of acephate is less than two
days in conditions anticipated by the U.S. EPA to occur from use.1
- In other laboratory studies, the calculated half-life of methamidophos
ranged from roughly one day in saturated silt loam soil to 13
days in half-saturated silt clay loam soil.5 The half-life of methamidophos
is expected to be less than ten days under use conditions
anticipated by the U.S. EPA.1
- Soil mobility of acephate and methamidophos is expected to be
high.1
- Field studies of acephate applied to crops found that acephate and
methamidophos were undetectable at 50 cm depths in silt loam,
sand, and loam soils for the duration of the study.1 Dissipation half-lives measured in these studies were two days or
less.1,6
- One study conducted on soil where tomatoes were grown found 11.3% of applied radiolabeled acephate had broken
down to methamidophos after one week, and it was the primary metabolite found.2
Water
- Despite acephates high solubility and mobility in water, acephate is considered a low risk of groundwater contamination
because acephate and methamidophos do not persist in the environment.1,46
- Acephate is not likely to volatilize from water because it is highly water soluble.4
- Acephate does not break down readily by photolysis in water. Only 1.6% broke down to methamidophos in this medium.4,6
- The half-life of acephate in the anaerobic clay sediment of a creek was between six and seven days. Acephate degraded
mostly to methane and carbon dioxide in this study.6
- Acephate hydrolysis is slow, but occurs faster at higher pHs.6 The half-life at pH 9 was 18 days. When mixed with distilled water
of pH levels 4-6 in laboratory conditions, 82-98% acephate was recovered from water after 20 days, and 0.2% of added
acephate was recovered as methamidophos.4
- Acephate was mixed with distilled water of pH 7.2, 8.1, and 8.6 and isolated in laboratory conditions at 37 °C for seven days.
About 40-60% of acephate was hydrolyzed mainly to O, S dimethyl phosphorothiolate within the first three days. After
three days decreases in acephate levels were small or unobservable.11 Methamidophos and O-methylacetyl phosphoramidothiolate
were minor products throughout this time course.11
- A product containing acephate was applied to three sites in a stream in British Columbia, Canada for five hours at levels
amounting to 1000 ppb.47 Acephate and methamidophos levels were measured in water and in river sediment at time
intervals up to 96 hours.47 Acephate levels detected in nearby sediment were highest four or eight hours after application
began, which was three or seven hours after peak concentrations of acephate were observed in the treated water.47 Methamidophos
was not detected in any of the water samples.47
Air
- Acephate is not volatile in ambient conditions.4 In one study, creek and pond water with added acephate were isolated in
flasks in laboratory conditions, and no acephate or methamidophos were detected from the air for up to fifty days.48
- At air saturation in environmental temperatures, acephate would be present in the air at 0.002 ppm.4
Plants
- Acephate was rapidly translocated and incorporated into plants when taken up from soil.2 Translocation occurs in the direction
of roots to foliage, not from foliage to roots.25
- In plants, acephate is primarily metabolized into methamidophos, which is a more potent insecticide. Methamidophos
found in leaves equaled 2-10% of the soil-applied acephate. These studies included tomatoes, beans, maize, fruit, spruce
trees and cones, Capsicum, and cucumber. No other metabolites were identified.2
- Acephate residues on treated leafy vegetables were highest on the outer leaves.25
- The half-life of acephate in tomatoes was 7-14 days.25
- Surface application of acephate to the peel of citrus fruit penetrated and distributed evenly into the fruit flesh.25
- Acephate applied to raspberry and cherry plants days before flowering resulted in residues of 0.15-2.84 ppm in nectar after
flowering. One raspberry plant's nectar contained 14.39 ppm acephate.49
Indoor
- No data regarding the indoor environmental fate of acephate were found.
Food Residue
- In 2008, 8686 samples of produce were tested for acephate.50 Four hundred ninety-one samples (5.7% of tested samples)
had detectable acephate residues.50 The majority of samples with acephate were celery or green beans.50 That year,
acephate was detected infrequently in spinach, summer squash, nectarines, blueberries, collard greens, strawberries, and
tomatoes.51
- In 2006, 8315 samples of produce were tested for acephate.51 Seventy-one samples (0.85% of tested samples) had detectable
acephate residues. Produce with detected acephate were mostly cranberries, spinach, or watermelon, and less frequently
winter squash, eggplant, and applesauce.51
- In 2008, 9694 samples of produce were tested for methamidophos. Four hundred thirty-nine samples, (4.5% of samples)
showed detectable levels of methamidophos.50 The majority of samples in which methamidophos was detected were
celery, green beans, or tomatoes.50 That year, methamidophos was detected infrequently in peaches, summer squash, asparagus,
nectarines, spinach, blueberries, and potatoes.51
- In 2006, 7524 samples of produce were tested for methamidophos.51 Ninety-four samples (1.25% of tested samples) had
detectable methamidophos residues. Produce with detected methamidophos most often were eggplant, watermelon, or
cranberries, and less frequently peaches, winter squash, spinach, frozen potatoes, frozen sweet peas, and applesauce.51
Birds
- Acephate is moderately toxic to birds. According to the U.S. EPA, oral LD50s ranged from 51-500 mg/kg in different bird species.1
One study found the LD50 for acephate in hens was 800 mg/kg.19
- Acephates degradation product, methamidophos, can be very highly toxic to birds on an acute basis. The LD50s for methamidophos
ranged from less than 10-50 mg/kg.1
- Blackbirds (Euphagus cyanocephalus), robins (Turdus migratorius), and starlings (Sternus vulgaris) approached lethality two
hours after oral exposure to 50-100 mg/kg acephate.26,52 The average LD50 for acephate in dark-eyed juncos (Junco hyemalis)
was 106 mg/kg, and for methamidophos was 8 mg/kg.26 Juncos given 26.52 mg/kg acephate by gavage showed brain
cholinesterase inhibition recovery between three and six days after treatment.26
- In multiple studies, most birds that died after acephate exposure had about 50% or greater brain cholinesterase inhibition.26,53,54
- Bobwhite quail (Colinus virginianus) were fed 80 ppm acephate in a chronic ingestion study. Body weight, number of eggs
laid, and embryo/hatchling viability were all reduced.1 In mallards (Anas platyrhyncos) chronically fed 20 ppm acephate, the
number of viable embryos and living embryos after three weeks was reduced.1 Egg thinning was observed in bobwhite
quail when parents were fed 5 ppm methamidophos on a chronic basis.1
- Field studies and incident reports indicated the potential for adverse effects to songbirds, including migratory pattern
disruption or death, after acephate field applications. In the field studies, adverse effects were delayed one or two days.1
Cholinesterase inhibition was observed in birds up to 33 days after 1.13 kg/ha treatment of a plot of land, and up to 26 days
after 0.57 kg/ha treatment.26,53
- In one study, white-throated sparrows (Zonotricia albicollis) were fed 256 ppm acephate in their food. Adult birds selected
random migratory directions compared to controls. Juvenile birds went south, whether or not they were fed acephate.55
Authors concluded acephate affected memory.55
- One study exposed white-throated sparrows to acephate in the diet over fourteen days. Results indicated an atypical dose
response relationship.18 At doses up to 16 ppm, the birds seemed to adapt to exposure by replenishing cholinesterase activity
in brain tissues. At doses between 16 and 1024 ppm, cholinesterase activity continued to decrease.18
Fish and Aquatic Life
- According to the U.S EPA, acephate is considered practically non-toxic to slightly toxic to freshwater fish for short-term
exposures. The LD50 ranges from 50 to greater than 100 ppm.1 In other studies, the 24-hour LC50 for rainbow trout ranged
from 900-2800 ppb.47 Up to tenfold bioaccumulation in bluegill sunfish (Lepomis macrochirus) was observed after fourteen
days of exposure to 0.007 and 0.7 ppm acephate.6
- Methamidophos is slightly toxic to freshwater fish for acute exposures with LD50s ranging from 10-100 ppm.1
- A product containing acephate was applied to a stream in British Columbia, Canada for five hours at levels amounting to
1000 ppb.47 While no fish deaths were observed, acephate and methamidophos were detected in fish in two out of three
sites up to eight hours after treatment.47 The percentage of methamidophos compared to acephate ranged from about
4-10%, when detected.47
- Acephate is practically non-toxic to slightly toxic in estuarine and marine fish for acute exposures; LC50 ranges from 50 to
greater than 100 ppm. Methamidophos is moderately toxic to estuarine or marine fish for acute exposures; LC50s range
from 1-10 ppm.1
- According to U.S. EPA sources, acephate is practically non-toxic to amphibians.1 In one study, a 24-hour LC50 of 6433 ppm
was reported for green frog (Rana clamitans) tadpoles. No behavioral changes were observed at 500 ppm acephate. In
another green frog tadpole study, no toxicity or bioaccumulation was seen at 5 ppm.6
- A salamander (Caudata) study identified a 96-hour LC50 of 8816 ppm acephate for salamander larvae.56 When salamander
egg masses were exposed to acephate at varying concentrations in the water, researchers observed muscle deformities
and bent vertebral columns at 382 mg/L, decreased growth, feeding, and lethargy at 798 mg/L, and the most sensitive
stage was the first week after hatching. Hatching time was not affected at the highest dose tested, 798 mg/L.56
- Acephate has low to moderate toxicity to aquatic invertebrates for acute exposures (LC50/EC50 ranged from 1 to greater
than 100 ppm).1 Methamidophos is very highly toxic to aquatic invertebrates for acute exposures (EC50 less than 0.1 ppm).1
See the text box on EC50.
EC50: The median effective concentration (EC50) may be
reported for sublethal or ambiguously lethal effects. This
measure is used in tests involving species such as aquatic
invertebrates where death may be difficult to determine.
This term is also used if sublethal events are being
monitored.
Newman, M.C.; Unger, M.A. Fundamentals of Ecotoxicology; CRC Press, LLC.:
Boca Raton, FL, 2003; p 178.
- Acephate affects daphnid reproduction, reducing the number of young. The lowest tested concentration that produced this
effect was 0.375 ppm. The daphnid No Observable Adverse Effect Concentration (NOAEC) was 0.150 ppm in this study.1
Terrestrial Invertebrates
- Acephate and methamidophos are highly toxic to bees and other beneficial insects. The LD50 for acephate was 1.2 μg/
honey bee. The LD50 of methamidophos was 1.37 μg/honey bee.1
- In one study, bee colonies were fed sugar syrup treated with 0.25, 0.5, or 1.0 ppm acephate for 14 days.49 At 0.25 ppm, the
number of eggs was reduced.49 At higher doses, some queens stopped laying eggs, others were lost.49 At 1 ppm levels,
bee flight activity and foraging were slowed.49 Over time, other effects were observed in all treatment groups, including
decreases in larvae and pupae rearing, newly built comb, feeding and food storage.49
- A product containing acephate was applied to three sites in a stream in British Columbia, Canada for five hours at levels
amounting to 1000 ppb.47 The highest level of acephate was 1189.5 ppb, detected in insects two and a half hours after the
application began. About two hours after the treatment, acephate was found in insects at only trace levels and was undetectable
at 24 hours.47 Acephate levels in insects were comparable to nearby water, about ten times higher than those
found in fish or sediment, and highest in stream bottom
insects compared to insects in cages.47 No insect deaths
were observed in this study.47
Reference Dose (RfD): The RfD is an estimate of the quantity of
chemical that a person could be exposed to every day for the rest
of their life with no appreciable risk of adverse health effects. The
reference dose is typically measured in milligrams (mg) of chemical
per kilogram (kg) of body weight per day.
U.S. Environmental Protection Agency, Integrated Risk Information System, IRIS Glossary, 2009. https://www.epa.gov/iris/iris-glossary#r
- The aPAD/aRfD is 0.005 mg/kg/day and the cPAD/cRfD
is 0.0012 mg/kg/day.1 See the text box on Reference Dose (RfD).
- The U.S. EPA has classified acephate as a Group C, possible human carcinogen. Increases in liver carcinomas and adenomas
were found in acephate studies using female mice.28 See the text box on Cancer.
- The Drinking Water Concentration of acephate that poses a risk level of one in 1,000,000 additional cancers is 4 μg/L.28
- The chronic RfD for acephate in rat studies was 4 x 10-3 mg/kg/day, based on brain cholinesterase inhibition.28
Date Reviewed: June 2011
Please cite as: Christiansen, A.; Gervais, J.; Buhl, K.; Stone, D. 2011. Acephate Technical Fact Sheet; National Pesticide
Information Center, Oregon State University Extension Services. https://npic.orst.edu/factsheets/archive/acephatech.html.



