1.800.858.7378npic@oregonstate.edu
Call, email, or chat Mon-Fri
A to Z
Paradichlorobenzene Technical Fact Sheet
As of 2011, NPIC stopped creating technical pesticide fact sheets. The old collection of technical fact sheets will remain available in this archive, but they may contain out-of-date material. NPIC no longer has the capacity to consistently update them. To visit our general fact sheets, click here. For up-to-date technical fact sheets, please visit the Environmental Protection Agency’s webpage.
- Chemical Class and Type
- Physical / Chemical Properties
- Uses
- Mode of Action
- Toxicity Classification
- Acute Toxicity
- Chronic Toxicity
- Endocrine Disruption
- Carcinogenicity
- Reproductive and Teratogenic Effects
- Fate in the Body
- Medical Tests and Monitoring
- Environmental Fate
- Ecotoxicity Studies
- Regulatory Guidelines
Chemical Class and Type:
- Paradichlorobenzene is a chlorinated aromatic hydrocarbon compound used as a fumigant insecticide and repellant.1 The International Union of Pure and Applied Chemistry (IUPAC) name for paradichlorobenzene is 1,4-dichlorobenzene. The Chemical Abstracts Service (CAS) registry number is 106-46-7.1
- Paradichlorobenzene was first registered for use in the United States by the United States Department of Agriculture (USDA) in 1947.1 See the text box on Laboratory Testing.
- There are three forms of dichlorobenzene, differing in the position of the two chlorine atoms on the benzene ring. 1,2-dichlorobenzene and 1,3-dichlorobenzene have been used to manufacture herbicides in addition to other products. 1,4-dichlorobenzene (paradichlorobenzene) is used as an insecticide.2 This fact sheet will address the para form unless otherwise noted.
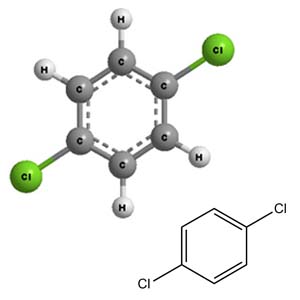
Molecular Structure -
Paradichlorobenzene
Laboratory Testing: Before pesticides are registered by the U.S. EPA, they must undergo laboratory testing for short-term (acute) and long-term (chronic) health effects. Laboratory animals are purposely given high enough doses to cause toxic effects. These tests help scientists judge how these chemicals might affect humans, domestic animals, and wildlife in cases of overexposure.
Physical / Chemical Properties:
- Paradichlorobenzene is a colorless to white solid crystal with a strong pungent odor.2,3 It will sublimate, turning from a solid directly into a gas, at room temperatures.3 The average sublimation rate over 19 days at 21-24 °C was 1.6-4.6 x 10-3 g/minute.4 Humans can detect paradichlorobenzene at airborne concentrations of 15 ppm.5
- Vapor pressure3: 4 x 10-1 mmHg at 20 °C
- Octanol-Water Partition Coefficient (log Kow) (unitless)6: 3.52
- Henry's constant (atm·m3/mol)2,6: 1.74 x 10-3 to 2.63 x 10-3
- Molecular weight (g/mol)6: C6H4Cl2 = 147.0
- Solubility (water)6: 0.08 g/L (80 mg/L) at 25 °C
- Soil Sorption Coefficient (Koc) (unitless)2: 275 to 833 depending on soil type
Uses:
- Paradichlorobenzene is primarily used in mothballs and similar products to protect clothing from moths.1 Uses for individual paradichlorobenzene products vary widely. Always read and follow the label when applying pesticide products.
- Paradichlorobenzene is also used in deodorant blocks for rest room toilets and trash containers.2
- Signal words for products containing paradichlorobenzene may range from Caution to Danger. The signal word reflects the combined toxicity of the active ingredient and other ingredients in the product. See the pesticide label on the product and refer to the NPIC fact sheets on Signal Words and Inert or "Other" Ingredients.
- To find a list of products containing paradichlorobenzene which are registered in your state, visit the website https://npic.orst.edu/reg/state_agencies.html select your state then click on the link for "State Products."
Mode of Action:
Target Organisms
- Paradichlorobenzene vapor is toxic to insects, molds, mildews, and it acts as a deodorizer.2,3 No information was found on the precise mode of action of paradichlorobenzene on target organisms.
Non-target Organisms
- The mode of action of paradichlorobenzene on non-target organisms is not precisely known, but may involve the binding of its oxidative metabolites such as the epoxide to proteins within the cells of mammals. Dichlorophenol, a product of epoxide hydrolysis, may be involved in nephropathy. Other metabolites such as quinones and hydroquinones may be responsible for the hepatotoxicity of paradichlorobenzene.3
- Recent work with paradichlorobenzene and human neuroblastoma SH-SY5Y cells in vitro suggested that paradichlorobenzene can act on the nicotinic acetylcholine receptor and appears to affect neuronal calcium ion homeostasis. Researchers noted that the concentrations of paradichlorobenzene observed to interfere with calcium homeostasis have been found in vivo in plasma, adipose, and liver tissues.7
Acute Toxicity:
Oral
LD50/LC50: A common measure of acute toxicity is the lethal dose (LD50) or lethal concentration (LC50) that causes death (resulting from a single or limited exposure) in 50 percent of the treated animals. LD50 is generally expressed as the dose in milligrams (mg) of chemical per kilogram (kg) of body weight. LC50 is often expressed as mg of chemical per volume (e.g., liter (L)) of medium (i.e., air or water) the organism is exposed to. Chemicals are considered highly toxic when the LD50/LC50 is small and practically non-toxic when the value is large. However, the LD50/LC50 does not reflect any effects from long-term exposure (i.e., cancer, birth defects or reproductive toxicity) that may occur at levels below those that cause death.
- The acute oral LD50 of paradichlorobenzene in male rats is 3863 mg/kg for males and 3790 mg/kg for females.8 Based on these values, paradichlorobenzene is low in toxicity via oral exposure.1 See the text boxes on Toxicity Classification and LD50/LC50.
- Paradichlorobenzene appears to be more toxic to mice than rats. A study using CD-1 mice identified an acute oral LD50 of 533 mg/ kg in males and 710 mg/kg in females.19 Researchers determined an LD50 of 353.6 mg/kg for female CD-1 mice based on an 8-day oral exposure.20
Dermal
- The acute LD50 for dermal exposure in rats was estimated to be greater than 6000 mg/kg for both males and females.8 Paradichlorobenzene is very low in toxicity via dermal exposure.1
- Paradichlorobenzene exposure in eye tissue led to conjunctivitis and corneal opacity in rabbits. Signs required up to 13 days to resolve following exposure. Based on total irritation scores, paradichlorobenzene was considered by the U.S. EPA to be a mild eye irritant.1,9
- Rabbits whose skin was exposed to paradichlorobenzene developed moderate to severe erythema, which persisted for 48 to 72 hours. Paradichlorobenzene is of low toxicity as a skin irritant.1,10
- Based on studies with guinea pigs, paradichlorobenzene is not a skin sensitizer.1,11
Inhalation
- The LC50 for acute inhalation exposure in rats was found to be greater than 6.0 mg/L, which was the highest exposure concentration that could be attained.12 The U.S. EPA considered paradichlorobenzene to be of low toxicity via inhalation exposure.1
Signs of Toxicity - Animals
- Companion animals who have eaten mothballs have suffered kidney and liver damage. Behavioral signs include abdominal pain, vomiting, seizures and tremors.13
- Birds exposed to paradichlorobenzene vapors may show weakness, head bobbing, anorexia, and depression.14 Signs of exposure in birds following ingestion of contaminated food or paradichlorobenzene itself include weight loss, immunosuppression, hepatitis, diarrhea, tremors, and seizures.15
Signs of Toxicity - Humans
- Paradichlorobenzene may be slightly irritating to the eyes and nasal passages if the vapor is inhaled.16 It can cause a burning sensation to the skin after prolonged contact.5
- Exposures in the workplace and in the home to high concentrations of paradichlorobenzene have resulted in fatigue, headache, nausea, vomiting, and weight loss. Other signs were ataxia, loss of coordination, weakness in arms and legs, slurred speech, and reduced reflexes.17,18
- A pregnant woman who ingested one or two paradichlorobenzene toilet air-freshener blocks per week for the first 38 weeks of her pregnancy developed refractory anemia with red blood cell damage and Heinz body formation. Symptoms included dizziness, swelling of the ankles, fatigue, and mild anorexia.19
- Acute hemolytic anemia has been reported following ingestion of moth crystals containing paradichlorobenzene.20
- A 69-year-old man developed allergic purpura (skin discoloration) after sitting in a chair treated with paradichlorobenzene crystals. The patient also developed acute glomerulonephritis.21
- Always follow label instructions and take steps to minimize exposure. If any exposure occurs, be sure to follow the First Aid instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 1-800- 222-1222. If you wish to discuss an incident with the National Pesticide Information Center, please call 1-800-858-7378.
Chronic Toxicity:
Animals
- Male and female rabbits, rats, and guinea pigs were exposed to 798 ppm paradichlorobenzene vapor for eight hours a day, five days a week for up to 69 days of exposure. Four of 16 rabbits, 2 of 23 guinea pigs, and 4 of 34 rats died during the exposure interval at unspecified times. Signs in exposed animals included weight loss, weakness, unkempt appearance,and tremors. Livers of treated animals exhibited central necrosis, and the kidneys of female rats exhibited swelling in the tubular epithelium.5
- Mice and rats were exposed to paradichlorobenzene vapor at 25, 55, 120, 270, and 600 ppm for 6 hours a day, five days a week for 13 weeks.22 Researchers observed liver hypertrophy in male rats and mice at the two highest doses, and in female rats and mice at the highest dose. Female mice exposed to 600 ppm paradichlorobenzene developed increases in mean blood cell hemoglobin. Male rats and mice in the 600 ppm exposure group developed significantly increased blood urea nitrogen, an indicator of muscle, blood, and protein breakdown. Male rats developed renal lesions and signs of nephropathy following exposure to 270 and 600 ppm paradichlorobenzene.22 The hepatoxicity of paradichlorobenzene was found to be greater in mice than in rats.22
- Researchers exposed 20 male rats, eight male guinea pigs, and eight female guinea pigs to 341 ppm paradichlorobenzene vapor for seven hours a day, five days a week, for six months. Some male guinea pigs exhibited fatty degeneration, cirrhosis, and focal necrosis in their livers. Exposed male guinea pigs also grew more slowly than control animals. The kidneys and livers of exposed male rats were heavier on average than the kidneys and livers of control animals.5
- Researchers also exposed five male and five female rats, five female and five male guinea pigs, and one male and one female rabbit to 173 ppm paradichlorobenzene vapor for seven hours a day, five days a week, for 16 days. None of the treated animals died, nor was their growth impaired relative to control animals. Liver and kidneys were heavier in exposed rats, and spleen weight in exposed male guinea pigs declined relative to control animals. Edema and alveolar hemorrhage were noted in the lungs of male rats, female guinea pigs and the female rabbit.5
- Ten male and female rats, 10 male mice, eight male and female guinea pigs, one male and female rabbit, and one female monkey were exposed to paradichlorobenzene vapor at concentrations of 158 ppm for 157 to 219 days. Liver weights of all exposed rats, female guinea pigs, and the kidney weights of exposed male rats increased. Exposed guinea pigs exhibited reduced growth rates compared to control animals.5
- Researchers dosed groups of two young male rats with either 10, 100, or 500 mg/kg paradichlorobenzene by gavage five days a week for four weeks. The highest dose produced swelling in the renal tubular epithelium, and necrosis and swelling in the liver lobules. No adverse effects were noted in the lower dose groups.5
- Researchers dosed 10 young female rats each with 18.8, 188, or 376 mg/kg paradichlorobenzene by gavage for five days a week, for a total of 138 doses in 192 days. Slight increases in the mean weight of the liver and kidneys occurred at the two highest dose rates, and the highest dose group also exhibited reduced spleen mass and focal necrosis and cirrhosis in the liver. No effects were observed in the low-dose group.5
Humans
- Workers at a manufacturing plant were exposed to up to 550 ppm of paradichlorobenzene in the course of their work. Workers were exposed eight hours a day, five days a week, for employment durations ranging from eight months to 25 years. No hematological effects or eye damage such as cataracts were noted during medical examinations.5 See the text box on Exposure.
- A 32-year-old woman reported consuming at least one paradichlorobenzene mothball per day for two years. Signs included dementia, difficulty speaking, ataxia (lack of coordination), hyperreflexia, distal limb weakness, and skin scaling. Blood plasma contained 34 μg/ml paradichlorobenzene.23
- Twin girls aged 18 years were hospitalized after sniffing paradichlorobenzene mothballs daily for 5-10 minutes per day for a few weeks in the case of one twin and 4-6 months in the other. Signs included intracranial hyptertension, unsteady gait, urinary retention, and neurological symptoms. Total recovery occurred after three and six months, respectively.24
Exposure: Effects of paradichlorobenzene on human health and the environment depend on how much paradichlorobenzene is present and the length and frequency of exposure. Effects also depend on the health of a person and/or certain environmental factors.
Endocrine Disruption:
- Researchers evaluated paradichlorobenzene's endocrine-disrupting potential using a yeast estrogen screen. Following nine days of incubation, paradichlorobenzene treatment caused dose-dependent increases in β-galactosidase production similar to 17β-estradiol, a human estrogen hormone.25
- In the same study, adult male and female zebrafish (Danio rerio) were exposed to 10, 18, 32, or 56 mg/L paradichlorobenzene for 96 hours. Other fish were exposed to 0.32, 1.0, 3.2, 10.0, or 32.0 mg/L 17α-ethynylestradiol (EE2), which was used as a positive control.25 Researchers estimated that paradichlorobenzene had a relative potency of 2.2 x 10-7 relative to 17β-estradiol.25
- Researchers exposed other adult male and female zebrafish to 1.0, 3.2, 10, and 32 mg/L paradichlorobenzene or 5, 10, 50, or 100 ng/L EE2 as a positive control for 2 weeks.25 Fish exposed to EE2 or 32 mg/L paradichlorobenzene exhibited edema in their peritoneal cavities and lacked developed gonads and intact eggs.25 Fish exposed to 10 or 32 mg/L paradichlorobenzene and all dose levels of EE2 also had significantly reduced gonad weight relative to overall body weight.25
Carcinogenicity:
Animals
- The National Toxicology Program concluded that there was "clear evidence of carcinogenicity" in male rats and both male and female B6C3F1 mice following gavage exposure to paradichlorobenzene.26
- Paradichlorobenzene appears to induce liver tumors through stimulation of precancerous lesions, via a mitogenic mode of action. Researchers concluded that paradichlorobenzene does not appear to be DNA reactive, genotoxic, or mutagenic.27
- Paradichlorobenzene's carcinogenic potential may result from inhibition of cell apoptosis. It does not appear to result from a genotoxic mechanism.28
- Researchers exposed groups of 50 male rats, 50 female rats, 50 male mice and 50 female mice per dose to paradichlorobenzene via inhalation exposure for six hours a day, five days a week, for two years. Treatment concentrations were 20, 75, or 300 ppm. Increased rates of liver cancers were noted in both sexes of mice exposed to 300 ppm paradichlorobenzene. No increased rates of tumor formation were noted in any of the rat treatment groups. Nasal lesions were considered the most sensitive endpoint following exposure.29
- In a two-year study conducted by the National Toxicology Program, mice were dosed with 300 or 600 mg/kg paradichlorobenzene by gavage five days a week for 103 weeks. Treated mice of both sexes developed non-neoplastic liver lesions and kidney abnormalities at greater rates than control mice. Liver cancers (hepatocellular neoplasms) increased in a dosedependent manner in both sexes of exposed mice.26
- F344/N rats were dosed with 150 or 300 mg/kg paradichlorobenzene by gavage five days a week for 103 weeks as part of the National Toxicology Program's assessment. Male rats developed kidney cancers (renal tubular cell adenocarcinomas) at dose-dependent rates. No such renal neoplasms were seen in female rats.26 Adult male rats form the protein á2u-globulin, which appears to have a major role in onset of nephropathy.30
Humans
- The Health Effects Division (HED) Cancer Assessment Review Committee (CARC) of the U.S. EPA has classified paradichlorobenzene
as not likely to be carcinogenic to humans.1 See the text box on Cancer.
Cancer: Government agencies in the United States and abroad have developed programs to evaluate the potential for a chemical to cause cancer. Testing guidelines and classification systems vary. To learn more about the meaning of various cancer classification descriptors listed in this fact sheet, please visit the appropriate reference, or call NPIC.
- The International Agency for Research on Cancer (IARC) of the World Health Organization concluded that paradichlorobenzene is possibly carcinogenic to humans, classifying it into Group 2B. The conclusion was based on greater incidences of liver tumors in exposed mice, and a suggested mechanism of carcinogenicity that could plausibly occur in humans.31
Reproductive or Teratogenic Effects:
Animals
- Rats and rabbits inhaled 50, 200, or 600 ppm for an unspecified period of time. At the two highest doses, food consumption and maternal body weight gain declined. Young born to animals dosed with 600 ppm displayed delayed cervical ossification.32
- Rabbits inhaled paradichlorobenzene vapors at 100, 300, or 800 ppm for an unspecified period of time. No teratogenic effects were noted, but mother rabbits in the highest dose group gained less weight than controls. Development of one of the major arteries on the aorta was altered in young born to the rabbits in the 800 ppm dose group.33
- Researchers performed a two-generation reproductive study on rats, exposing both males and females to 50, 150 or 450 ppm paradichlorobenzene vapor for an unspecified period of time. The high-dose group produced fewer live pups that weighed less and had greater mortality rates during the first four days following birth. Male rats showed evidence of nephropathy in the lowest-dose group, although this was considered an effect that is unique to male rats due to their physiology.34
Humans
- A woman who ingested one or two toilet air freshener blocks per week made of paradichlorobenzene for the first 38 weeks of her pregnancy gave birth to a normal baby girl. No blood abnormalities were found in either the mother or the child at the time of delivery although the mother suffered from anemia at the time of hospital admission at 38 weeks.19
Fate in the Body:
Absorption
- Paradichlorobenzene is absorbed rapidly through the lungs and gastrointestinal tract. Researchers demonstrated that absorption of paradichlorobenzene is more rapid via the oral route than by inhalation in mice. Absorption rates may also vary by species.3
- Seven human volunteers inhaled paradichlorobenzene at concentrations of 2.4-2.8 ppm for one hour. Researchers estimated that 46-67% of the inhaled paradichlorobenzene was absorbed by the lungs.35
- Dermal absorption of paradichlorobenzene is slow.3
Distribution
- Paradichlorobenzene residues have been found in human blood, fat, and breast milk.36,37,38 The metabolite 2,5-dichlorophenol (DCP) has been found in human urine.36
- Distribution varied by gender in rats exposed to paradichlorobenzene vapor at concentrations of 500 ppm for 24 hours. Male rats had significantly lower concentrations of paradichlorobenzene in their livers than females, whereas females had significantly lower concentrations in their kidneys. Concentrations peaked at 3 hours following termination of the exposure period and declined to very low levels by 24 hours post-exposure in both organs for both genders.30
- Researchers exposed female rats via inhalation exposure to paradichlorobenzene for 10 days at 1000 ppm for three hours per day. Tissue concentrations peaked at six days of exposure. At that time, the greatest concentrations were found in the fat, with 597 ppm paradichlorobenzene detected. Much lower concentrations were present in the kidneys (43 ppm) and liver (28 ppm). Blood plasma, muscle and lungs contained maximum concentrations of 19, 7 and 11 ppm paradichlorobenzene, respectively.39
- Fischer 344 rats were orally dosed with paradichlorobenzene at concentrations of either 100 or 1000 mg/kg in a single exposure.40 Blood plasma concentrations of paradichlorobenzene and its metabolite 2,5-dichlorophenol fell below detectable limits one day after exposure in the low-dose group. Four days after exposure, only traces of 2,5-dichlorophenol were detected in blood plasma in the high-dose group.40 Paradichlorobenzene and 2,5-dichlorophenol were not detected in adipose and liver tissue four days post-dose, although the kidney still had traces of 2,5-dichlorophenol.40
- Researchers fed paradichlorobenzene to Fischer 344 rats at concentrations of 100 or 1000 ppm for 28 days. Animals were then given clean food for an additional seven days.40 No paradichlorobenzene or its metabolites were detected in the lowdose group. Blood plasma levels peaked at day three then declined rapidly, presumably because of enzyme induction.40 Adipose, liver, and kidney tissue residues showed similar patterns, and no traces of paradichlorobenzene or 2,5-dichlorophenol were detected at day 35.40
- Researchers exposed Wistar rats to paradichlorobenzene vapors at concentrations of 75 or 500 ppm (450 and 3000 mg/ m3) for five hours a day, five days a week, for 18 months. Rats were sacrificed and necropsied at six months, 18 months (the termination of exposure), and six months after the exposure ended.40 Paradichlorobenzene was found at trace levels in the plasma of the high-dose group after six months of exposure.40 At six months, 2,5-DCP was measured at concentrations proportional to the dose of paradichlorobenzene, but concentrations were much reduced at 18 months in the high-dose group.
- In the study above, adipose tissue concentrations of paradichlorobenzene changed between six to 18 months of exposure. Changing tissue concentrations varied by sex. In the low-dose group, males had lower levels of paradichlorobenzene in adipose tissue than females, and in the high-dose group, females had lower levels than males. In both dose groups and both sexes, levels in the adipose tissue decreased over time. The livers of low-dose animals contained traces of paradichlorobenzene and 2,5-DCP at 6 months but no detectable traces after 18 months. The livers from the high-dose group also contained greater concentrations of paradichlorobenzene and 2,5-DCP at six months than at 18 months. All liver concentrations were below 6 μg/g.40
Metabolism
- The main metabolite of paradichlorobenzene in humans is 2,5-dichlorophenol, but 2,5-dichlorohydroquinone, 2,5-dichlorophenylmethyl sulfide, 2,5-dichlorophenylmethyl sulfoxide, 2,5-dichlorophenylmethyl sulfone and mercapturic acid derivatives have also been detected in urine.35
- Adult male Wistar rats dosed with 10, 50, or 250 mg/kg paradichlorobenzene by gavage excreted the sulfate and glucuronide forms of 2,5-dichlorophenol in their urine. These metabolites accounted for 70% of the dose.41 Mercapturic acid compounds derived from glutathione conjugates accounted for 10% of the original dose. 2,5-dichlorophenol was also detected.41
- Rats were dosed orally via gavage with radio-labeled paradichlorobenzene at 900 mg/kg. After 72 hours, major metabolites detected were 2,5-dichlorophenol in the form of sulfide and gulcuronide conjugates.42 No sex-specific differences were noted.42
- Researchers exposed male and female Fischer 344 rats to 150 or 600 mg/kg paradichlorobenzene by gavage once a day for up to 28 days.40 Phase I enzymes P450 CYPA1, CYP2B1, and CYP2D1 were induced, reaching maximum levels on day nine. Enzyme levels in female livers were greater than in male livers.40 The enzymes CYP2B1, CYP3A1, and CYP3A4 were induced in female rats but not in males.40 The phase II enzymes epoxide hydrolase and glutathione S-transferase peaked at day nine, but only the high-dose groups showed substantial induction. Induction of phase II enzymes occurred more strongly in male rat livers than in females. Enzyme induction in the kidneys was much lower than in the liver.40
Excretion
- Human volunteers who inhaled 2.4-2.8 ppm paradichlorobenzene exhaled 0.9-1.4 ppm paradichlorobenzene during the exposure period of one hour. Exhaled paradichlorobenzene dropped below the detection limit of 0.02 ppm within 10 minutes of the end of exposure.35
- In the same study, the volunteer subjects excreted 4.8-16.2% of the absorbed dose of paradichlorobenzene as 2,5-dichlorophenol in their urine within 10 hours of the end of the one-hour exposure.35 Blood serum concentrations of paradichlorobenzene dropped by at least 50% during the first hour post-exposure.35
- Adult male Wistar rats were dosed with 10, 50, or 250 mg/kg of paradichlorobenzene by gavage. Some of the rats were first pre-treated with isoniazid to induce cytochrome P450 2E1 activity. Clearance rates and half-lives of paradichlorobenzene were not dose-dependent. Rats with induced enzyme activity had greater clearance rates and reduced blood half-lives of paradichlorobenzene. The half-life of paradichlorobenzene in blood ranged from 7.06 to 8.12 hours.41 Eighty percent of the radiolabeled dose was excreted in urine and 4% was excreted in feces. Urinary excretion was enhanced by cytochrome P450 2E1 induction. Bile was a minor elimination pathway, accounting for 4-30%.41
- In another study, researchers dosed rats orally via gavage with radio-labeled paradichlorobenzene at 900 mg/kg. After 72 hours, male rats had excreted 41.3% and females 37.8% of the radio label in their urine.42
Medical Tests and Monitoring:
- Biomarkers of exposure to paradichlorobenzene and its metabolite 2,5-dichlorophenol have been reported in the scientific literature. Scientists have used a purge and trap apparatus followed by gas chromatography and mass spectrometry to detect and quantify paradichlorobenzene in blood.36,43 Capillary gas chromatography and mass spectrometry have been used to quantify 2,5-dichlorophenol in urine.36,44,45 These methods of testing for exposure to paradichlorobenzene and its metabolites have not been well-studied in humans, and the clinical significance is currently unknown.46
- Researchers analyzed urine or blood from 1,000 adults throughout the United States as part of the third National Health and Nutrition Examination Survey. They detected 2,5-dichlorophenol, the major metabolite of paradichlorobenzene, in 98% of urine and 96% of blood samples evaluated.36
- Paradichlorobenzene was detected in the blood of 41-89% of children tested during four separate monitoring sessions involving a total of 134 children in the third National Health and Nutrition Examination Survey.47 In another study, the metabolite 2,5-dichlorophenol was found in the urine of 96% of the 197 children tested, at median concentrations of 9 μg/g and a maximum of 1200 μg/g.48
Environmental Fate:
Soil
The "half-life" is the time required for half of the compound to break down in the environment.
1 half-life = 50% remaining
2 half-lives = 25% remaining
3 half-lives = 12% remaining
4 half-lives = 6% remaining
5 half-lives = 3% remaining
Half-lives can vary widely based on environmental factors. The amount of chemical remaining after a half-life will always depend on the amount of the chemical originally applied. It should be noted that some chemicals may degrade into compounds of toxicological significance.
- Volatilization is considered the dominant pathway of removal for paradichlorobenzene in soil. Much less of the compound is removed via photolysis, hydrolysis, oxidation, or biodegradation.2
- Proposed breakdown products for any paradichlorobenzene that does not volatilize include 1,4-dichlorobenzene dihydrodiol, 2,5-dichloro-cis,cis-muconic acid, 2-chloromaleyl-acetic acid, and 2-chloroaceto-acrylic acid.2
- Half-lives of paradichlorobenzene in soil are increased if conditions do not favor volatilization.2 See the text box on Halflife.
- Researchers spiked sandy loam soil with either sewage sludge or a mixed standardized solution of chlorobenzene compounds. Samples were taken at 0, 0.6, 1.6, 3.6, 7.6, 18, 32, 67, 147, and 259 days.49 Volatilization was responsible for most of the losses of chlorobenzenes from soil.49 The overall half-lives were estimated to be 22.1 days for sewage-amended soil and 11.2 days for soil spiked with chlorobenzenes.49 The authors cautioned that laboratory conditions likely reduced soil half-life compared to likely field conditions.49
- A species of Pseudomonas bacteria isolated from sewage sludge metabolized paradichlorobenzene to 3,6-dichloro-cis-1,2-dihydroxycyclohexa- 3,5-diene, and then to 3,6-dichlorocatechol. This in turn was metabolized to 2,5-dichloro-cis, cis-muconate. This species of Pseudomonas was able to draw all of its energy and carbon requirements from paradichlorobenzene.50
Water
- Researchers concluded from measurements of paradichlorobenzene in the waters of Lake Zurich that losses to the atmosphere accounted for the majority of elimination from the lake system.51
- Adsorption to aquatic sediments may be a dominant fate process in some systems and may decrease volatilization. Biodegradation may occur under aerobic conditions.52 Paradichlorobenzene does not appear to be broken down by bacteria under anoxic conditions.53 Photolysis, oxidation, and hydrolysis do not appear to be major processes of degradation for paradichlorobenzene in water.52
- Under aerobic conditions, biofilm that had been previously exposed to paradichlorobenzene for 10 days eliminated 98% of the chemical.54
- Paradichlorobenzene has been detected in a small percentage of samples of drinking water, surface water, and ground water throughout the United States. Detected concentrations were in the parts per trillion to parts per billion range.2,52,55
- Paradichlorobenzene concentrations in groundwater declined with distance from the source river, but the rate of decline appeared to be seasonal. Researchers speculated that paradichlorobenzene was more likely to penetrate the aquifer during periods of anoxic conditions within the aquifer.56
- Municipal wastewaters from six treatment plants in southern California were sampled in July and December in 1975. Concentrations of paradichlorobenzene ranged from less than 0.4 to 230 μg/L.57 Researchers estimated that 4,680 kg/yr paradichlorobenzene were released from outfall zones. Sediments collected 2 km down-current from outfall zones contained less than 0.019 mg/kg dry weight paradichlorobenzene.57
Air
- Paradichlorobenzene sublimes at room temperatures, transforming from a solid directly into a gas.2
- Paradichlorobenzene appears to be broken down primarily by interaction with hydroxyl radicals in the atmosphere. The reaction rate has been reported as 3.2-4.9 x 10-13 cm3/mol-sec.58 The half-life of paradichlorobenzene in the atmosphere has been estimated at 31 days.52
- Paradichlorobenzene has been detected in rainwater during storms at concentrations of 3.3-7.0 ng/L at ground level in a residential neighborhood of Portland, Oregon.59
Plants
- Carrots were grown in planters containing one of four soil treatments. Soil was amended with either low (6.35 g/kg dry weight) or high (59.5 g/kg dry weight) concentrations of sewage sludge containing paradichlorobenzene or spiked with paradichlorobenzene alone at 59.5 g/kg dry weight.60 Carrot foliage from plants grown in control soil, spiked soil, low sewage sludge, and high sewage sludge treatments contained 13 ± 1.2 (mean ± SD), 17 ± 4.0, 22 ± 0.43, and 49 ± 3.9 μg/kg paradichlorobenzene per dry weight of soil.60 Detected levels of paradichlorobenzene in carrot roots ranged from 5.8 μg/ kg to 9.6 μg/kg from treated and untreated soils. Researchers hypothesized that foliar uptake of volatilized paradichlorobenzene could have been an important pathway.60
- Samples of Chinese cabbages, carrots, celery, radishes, and spinach were obtained from three sites near an urban area in China. Researchers also collected soil from each site.61 Paradichlorobenzene was detected in spinach and cabbage stems and roots, in the leaves and roots of celery, and the roots of radishes. The highest residues were in celery, with 89 μg/kg paradichlorobenzene in the stems and roots. Researchers did not detect paradichlorobenzene in carrots, and it was present only in the roots of radishes from one site. Soil samples taken from each of the three sites contained an average of 4-27 μg/kg paradichlorobenzene.61
- The aquatic macrophyte Ceratophyllum demersum was exposed to paradichlorobenzene at concentrations of 0.5, 1, 5, 10 and 20 mg/L for 24 hours. At 10 mg/L, researchers observed increases in soluble glutathione S-transferase, glutathione reductase activity, and glutathione peroxidase activities. The researchers speculated that C. demersum biotransformed paradichlorobenzene via conjugation to glutathione, and that adverse oxidative effects could occur from paradichlorobenzene exposure.62
Indoor
- Reported mean concentrations of paradichlorobenzene in indoor air have ranged from 0.65 μg/m3 to 30 μg/m3 and may be affected by sampling method, indoor environmental conditions and other factors.63,64,65
- Indoor concentrations of paradichlorobenzene were reported from bathrooms and bedrooms where deodorizers and moth flakes containing paradichlorobenzene were used. Reported concentrations inside closets treated with moth flakes ranged from 219 to 545 ppb. Concentrations outside of the closets where moth flakes were used ranged from 10.3 to 71.0 ppb. In bathrooms where paradichlorobenzene deodorizers were used, detected concentrations ranged from 78 to 220 ppb.4
- Emission rates of paradichlorobenzene from commercial moth repellents varied even in products of similar size and shape, although emission rates increased exponentially with increases in ambient temperature. Steady-state concentrations of paradichlorobenzene in four styles of clothing storage containers were reached at the end of the first week, but the concentration of paradichlorobenzene retained in the containers varied widely, as did the amount of paradichlorobenzene that leaked into ambient air around the storage cases.66
- Clothing stored with paradichlorobenzene mothballs may absorb over 900 μg/g of the chemical after five days depending on where the clothing is placed relative to the mothballs. Although one hour of airing after removal from the storage container reduced these residues, about half of the residue remained. Researchers concluded that clothing stored with paradichlorobenzene could itself be a source of indoor air contamination.67
Food Residue
- Paradichlorobenzene was detected in a wide range of prepared foods during the U.S. Food and Drug Administration's (FDA) Total Diet Study, which included analysis of market basket samples from 1991 to 2003. Of the 51 prepared foods analyzed for paradichlorobenzene, 29 foods had one or more samples with levels above the limit of quantification. Mean levels of paradichlorobenzene detected ranged from 0.05 ppb (natural cheddar cheese) to 0.0475 ppm (sunflower seeds). Foods with the three highest maximum levels detected were: popcorn popped in oil (0.292 ppm), salted margarine (0.208 ppm), and roasted, salted sunflower seeds (0.190 ppm).68
- Researchers evaluated fresh vegetables purchased at retail outlets in the United Kingdom for the presence of various chlorobenzenes. Paradichlorobenzene was detected in 61% (22 of 36) of samples analyzed. Vegetables evaluated included: carrots, potatoes, cabbages, cauliflowers, lettuces, onions, beans, peas, and tomatoes. Bean seeds (0.717 μg/kg fresh weight) and tomato peels (0.619 μg/kg fresh weight) had the highest levels detected, whereas bean pods (0.117 μg/kg fresh weight) and outer lettuce leaves (0.118 μg/kg fresh weight) had the lowest amounts detected.69
- Paradichlorobenzene has been detected in various fish and aquatic organisms consumed by humans. Trout from the U.S. Great Lakes region had levels of paradichlorobenzene ranging from 1 to 4 ppb.70
- Paradichlorobenzene was detected in the lipid tissue of fish and crabs caught in the Calcasieu River Estuary near the outfall of industrial facilities near Lake Charles, LA. Levels in catfish ranged from 0.17 to 0.47 μg/g of lipid, depending on the location caught. Levels of paradichlorobenzene in other fish ranged from 0.24 to 0.90 μg/g of lipid. The highest amount of paradichlorobenzene detected was in the lipid tissue of blue crabs (Callinectes sapidus) at 2.5 μg/g.71
- Crabs (Charybdis feriatus) contained paradichlorobenzene at concentrations of 0.5, 0.6 and 5.1 μg/kg in the leg meat, body meat and carapace meat, respectively.72
Ecotoxicity Studies:
Birds
- Researchers fed 10 young Peking ducks 0.5% paradichlorobenzene in their diet for 35 days. By the end of day 28, three ducks had died and the survivors showed decreased growth rates.5
Fish and Aquatic Life
- Researchers exposed rainbow trout (Oncorhyncus mykiss) and zebrafish (Danio rerio) to water treated with paradichlorobenzene.
The 48-hour LC50 for rainbow trout was 1.18 mg/L, and the 48-hour LC50 for zebrafish was 4.25 mg/L.73
EC50: The median effective concentration (EC50) may be reported for sublethal or ambiguously lethal effects. This measure is used in tests involving species such as aquatic invertebrates where death may be difficult to determine. This term is also used if sublethal events are being monitored.
Newman, M.C.; Unger, M.A. Fundamentals of Ecotoxicology; CRC Press, LLC.: Boca Raton, FL, 2003; p 178.
- In the same study, researchers exposed a species of green algae (Selenastrum capricornutum) and an aquatic invertebrate (Daphnia magna) to paradichlorobenzene in water. The 96-hour EC50 for growth inhibition of the algae was 1.6 mg/L, and the three-hour EC50 for photosynthesis reduction was 5.2 mg/L. For D. magna, the 24-hour immobilization concentration (IC50) was 1.6 mg/L.73 See the text box on EC50.
- Sheepshead minnows (Cyprinodon variegates) were exposed to paradichlorobenzene in water. Mortality was recorded at 24, 48, 72 and 96 hours. The LC50 values ranged from greater than 7.5 to 10.0 ppm for 24-hour tests and 7.2-7.4 ppm for 48- to 96-hour tests.74
- Researchers exposed zebrafish (Danio rerio) to paradichlorobenzene in 96-hour static exposure tests, and estimated the acute LC50 to be 22 mg/L.25
- The LC50 of paradichlorobenzene to bluegill sunfish (Lepomis macrochirus) was determined to be 4.5 mg/L for 24 hours and 4.3 mg/L for 96 hours, but some of the paradichlorobenzene was seen to precipitate out of solution.75 This likely led to an overestimate of the LC50.
- The 96-hour LC50 in fathead minnows (Pimephales promelas) has been reported as 2.85, 30.0, and 33.7 mg/L.76
- Muscle tissue from six Dover sole (species not specified) captured at a site 2 km down-current from the outfall of a municipal sewage treatment plant contained less than 0.007 mg/kg wet weight paradichlorobenzene. Sediments from the same site contained less than 0.019 mg/kg dry weight paradichlorobenzene.57
- Lake trout (Salvelinus namaycush) were collected from Lakes Ontario, Superior, and Huron and rainbow trout (Oncorhyncus mykiss) were collected from Lake Erie. All fish were estimated to be at least four years old and they contained concentrations of paradichlorobenzene ranging from 1-4 ppb.55
- Juveniles of the marine polychaete worm (genus Neanthes, species unspecified) grew significantly less near a sewage outfall. Sediments at sampling sites contained a range of contaminants including paradichlorobenzene.77 Subsequent laboratory studies attempted to replicate the reduced growth rates by exposing juvenile Neanthes to clean sediments spiked with paradichlorobenzene at concentrations an order of magnitude greater than those measured in the field. Significant reductions in dry weight were detected only at the highest concentration tested. Researchers speculated that paradichlorobenzene alone was not responsible for effects observed in the field.76
- The LC50 for juvenile sand crabs (Portunus pelagicus) was estimated to be 5.03 μmol/L.78,79 The EC10 and EC50, defined by the researchers as concentrations of paradichlorobenzene at which growth rate was reduced by 10% and 50% compared to controls, was estimated to be 0.44 and 1.37 μmol/L, respectively.79
- Researchers exposed green alga (Ankistrodesmus falcatus) to paradichlorobenzene and estimated the EC50 to be 0.136 mM paradichlorobenzene. At that concentration, primary productivity of the alga was reduced by 50%.80
Terrestrial Invertebrates
- No information was found regarding the toxicity of paradichlorobenzene to terrestrial invertebrates.
Regulatory Guidelines:
Maximum Contaminant Level (MCL): The MCL is the highest level of contaminant that is legally allowed in drinking water. The MCL is enforceable. The MCL is typically measured in milligrams (mg) of contaminant per liter (L) of water.
U.S. Environmental Protection Agency, National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#one
- The Health Effects Division (HED) Cancer Assessment Review Committee (CARC) of the EPA has classified paradichlorobenzene as not likely to be carcinogenic to humans.1 See the text box on Cancer.
- The concentration of paradichlorobenzene considered Immediately Dangerous to Life and Health (IDLH) is 150 ppm.81
- PEL/TWA: The Permissible Exposure Level for a time-weighted average exposure (PEL TWA) for paradichlorobenzene is 10 ppm.82
- The Maximum Contaminant Level (MCL) for paradichlorobenzene in drinking water is 0.075 mg/L or 75 ppb.83 See the text box on Maximum Contaminant Level (MCL).
- The Threshold Limit Value-Time Weighted Average (TLV-TWA) is 10 ppm for paradichlorobenzene.2
- The Minimal Risk Level (MRL) for acute inhalation exposure to paradichlorobenzene has been set at 2 ppm.2
Please cite as: Gervais, J.; Luukinen, B.; Buhl, K.; Stone, D. 2010. Paradichlorobenzene Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services. https://npic.orst.edu/factsheets/archive/PDBtech.html.
References:
- Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Toxicological profile for dichlorobenzenes; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, 2006.
- Para-Dichlorobenzene: HED chapter of the Reregistration Eligibility Decision Document (RED); U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office : Washington, DC, 2007.
- Scuderi, R. Determination of para-dichlorobenzene releases from selected consumer products. Unpublished report, 1986, submitted to U.S Department of Health and Human Services prepared by Midwest Research Institute, Kansas City, MO. Toxicological profile for dichlorobenzenes; U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, 2006.
- Hollingsworth, R. L.; Rowe, V. K.; Hoyle, H. R.; Spencer, H. C. Toxicity of paradichlorobenzene. AMA Arch. Ind. Health 1956, 14 (2), 138-147.
- Lide, D. CRC Handbook of Chemistry and Physics; CRC Press Inc.: Boca Raton, FL, 1994; p 16-25.
- Yan, R. M.; Chiung, Y. M.; Pan, C. Y.; Liu, J. H.; Liu, P. S. Effects of dichlorobenzene on acetylcholine receptors in human neuroblastoma SH-SY5Y cells. Toxicol. 2008, 253 (1-3), 28-35.
- Gaines, T. B.; Linder, R. E. Acute toxicity of pesticides in adult and weanling rats. Fundam. Appl. Toxicol. 1986, 7, 299-308.
- Morris, T. Primary eye irritation study in rabbits: para-dichlorobenzene. Unpublished lab project no. 91-8305-21(B), 1992, submitted to U.S. Environmental Protection Agency prepared by Hill Top Biolabs, Inc. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Morris, T. Primary skin irritation study in rabbits: para-dichlorobenzene. Unpublished lab project no. 91-8305- 21(A), 1992, submitted to U.S. Environmental Protection Agency prepared prepared by Hill Top Biolabs, Inc. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Morris, T. Delayed contact hypersensitivity in guinea pigs (Buehler technique): para-dichlorobenzene. Unpublished lab project no. 9-8305-21(C), 1992, sumbitted to U.S. Environmental Protection Agency prepared by Hill Top Biolabs, Inc. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Newton, P. An acute inhalation toxicity study of para-dichlorobenzene in the rat. Unpublished lab project no. 89- 8230, 1990, submitted to U.S. Environmental Protection Agency prepared by Bio/dynamics, Inc. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Oehme, F. W.; Kore, A. M. Miscellaneous indoor toxicants. Small Animal Toxicology, 2nd ed.; Peterson, M. E.; Talcott, P. A. Eds.; Elsevier: St. Louis, MO, 2006; p 240.
- DeClementi, C. Moth repellent toxicosis. Vet. Med. 2005, 24-28.
- LaBonde, J. Toxicity in pet avian patients. Semin. Avian Exot. Pet Med. 1995, 4 (1), 23-31.
- Reigart, J. R.; Roberts, J. R. Fumigants. Recognition and Management of Pesticide Poisonings, 5th ed.; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 1999; p 160.
- Cotter, L. H. Paradichlorobenzene poisoning from insecticides. N. Y. State J. Med. 1953, 53 (14), 1690-1692.
- Miyai, I.; Hirono, N.; Fujita, M.; Kameyama, M. Reversible ataxia following chronic exposure to paradichlorobenzene. J. Neurol. Neurosurg. Psychiatr. 1988, 51, 453-454.
- Campbell, D. M.; Davidson, 19. R. J. L. Toxic haemolytic anaemia in pregnancy due to a pica for paradichlorobenzene. J. Obstet. Gynaecol. Br. Commonw. 1970, 77, 657-659.
- Hallowell, M. Acute haemolytic anemia following the ingestion of para-dichlorobenzene. Arch. Dis. Child. 1959, 34, 74-75.
- Nalbandian, R. M.; Pearce, J. F. Allergic purpura induced by exposure to p-dichlorobenzene. J. Am. Med. Assoc. 1965, 194 (7), 828-829.
- Aiso, S.; Arito, H.; Nishizawa, T.; Nagano, K.; Yamamoto, S.; Matsushima, T. Thirteen-week inhalation toxicity of p-dichlorobenzene in mice and rats. J. Occup. Health 2005, 47, 249-260.
- Kumar, N.; Dale, L. C.; Wijdicks, E. F. M. Mothball mayhem: relaping toxic leukoencephalopathy due to p-dichlorobenzene neurotoxicity. Ann. Intern. Med. 2009, 150 (5), 362-363.
- Feuillet, L.; Stephanie, M.; Spadari, M. Twin girls with neurocutaneous symptoms caused by mothball intoxication. N. Engl. J. Med. 2006, 35 (4), 423-424.
- Versonnen, B. J.; Arijs, K.; Verslycke, T.; Lema, W.; Janssen, C. R. In vitro and in vivo estrogenicity and toxicity of o-, m-, and p-dichlorobenzene. Environ. Toxicol. Chem. 2003, 22 (2), 329-335.
- NTP Toxicology and Carcinogenesis Studies of 1,4-Dichlorobenzene in F344/N Rats and B6C3F1 Mice; U.S. Departmenet of Health and Human Services, National Institutes of Health. http://ntp-server.niehs.nih.gov/htdocs/LT_rpts/tr319.pdf (accessed Feb 2010), updated Jan 1987.
- Butterworth, B. E.; Aylward, L. L.; Hays, S. M. A mechanism-based cancer risk assesment for 1,4-dichlorobenzene. Regul. Toxicol. Pharmacol. 2007, 49, 138-148.
- Kokel, D.; Li, Y.; Qin, J.; Xue, D. The nongenotoxi carcinogens naphthalene and para-dichlorobenzene suppress apoptosis in Caenorhabditis elegans. Nat. Chem. Biol. 2006, 2 (6), 38-345.
- Aiso, S.; Takeuchi, T.; Arito, H.; Nagano, K.; Yamamoto, S.; Matsushima, T. Carcinogenicity and chronic toxicity in mice and rats exposed by inhalation to para-dichlorobenzene for two years. J. Vet. Med. Sci. 2005, 67 (10), 1019-1029.
- Umemura, I.; Takada, K.; Ogawa, Y.; Kamata, E.; Saito, M.; Kurokawa, Y. Sex difference in inhalation toxicity of p-dichlorobenzene (p-DCB) in rats. Toxicol. Lett. 1990, 52, 209-214.
- Dichlorobenzenes. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, International Agency for Research on Cancer: Lyon, France, 1999; Vol. 73, pp 223-265.
- Neeper-Bradley, T.; Kubena, F. Developmental toxicity study of maternally inhaled paradichlorobenzene vapor in CD rats. Unpublished lab project no. 91N0110, 1992, submitted to U.S. Environmental Protection Agency prepared by Union Carbide Chemicals and Plastics Company, Inc. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Hayes, W.; Gushow, T.; John, J. para-Dichlorobenzene: inhalation teratology study in rabbits. Unpublished report file no. k-1323-(12), 1982, sumbitted to U.S. Environmental Protection Agency prepared by Dow Chemicals USA. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Tyl, R.; Neeper-Bradley, T. Paradichlorobenzene: two-generation reproduction study of inhaled paradichlorobenzene in Sprague-Dawley (CD) rats. Unpublished lab project no. 86-81-90606, 1989, submitted to U.S. Environmental Protection Agency prepared by Union Carbide Bushy Run Research Center. Revised Reregistration Elegibility Decision (RED) Para-dichlorobenzene, EPA 738-R-07-010; U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Office of Pesticide Programs, U.S. Government Printing Office: Washington, DC, 2008.
- Yoshida, T.; Andoh, K.; Kosaka, H.; Kumagai, S.; Matsunaga, I.; Akasaka, S.; Nakamura, S.; Oda, H.; Fukuhara, M. Inhalation toxicokinetics of p-dichlorobenzene and daily absorption and internal accumulation in chronic low-level exposure to humans. Arch. Toxicol. 2002, 76, 306-315.
- Hill, R. H., Jr.; Ashley, D. L.; Head, S. 36. L.; Needham, L. L.; Pirkle, J. L. p-Dichlorobenzene exposure among 1,000 adults in the United States. Arch. Environ. Health 1995, 50 (4), 277-280.
- Morita, M.; Ohi, G. Para-dichlorobenzene in human tissue and atmosphere in Tokyo metropolitan area. Environ. Pollut. 1975, 8, 269-273.
- Jan, J. Chlorobenzene residues in human fat and milk. Bull. Environ. Contam. Toxicol. 1983, 30, 595-599.
- Hawkins, D. R.; Chasseaud, L. F.; Woodhouse, R. N.; Cresswell, D. G. The distribution, excretion, and biotransformation of p-dichloro[14C]benzene in rats after repeated inhalation, oral and subcutanous doses. Xenobiotica 1980, 10 (2), 81-95.
- Bomhard, E. M.; Schmidt, U.; Loser, E. Time course of enzyme induction in liver and kidneys and absorption, distribution and elimination of 1,4-dichlorobenzene in rats. Toxicol. 1998, 131, 73-91.
- Hissink, A. M.; Dunnewijk, R.; van Ommen, B.; van Bladeren, P. J. Kinetics and Metabolism of 1,4-Dichlorobenzene in Male Wistar Rats: No Evidence for Quinone Metabolites. Chem. Biol. Interact. 1997, 103, 17-33.
- Klos, C.; Dekant, W. Comparative Metabolism of the Renal Carcinogen 1,4-Dichlorobenzene in Rat: Identification and Quantitation of Novel Metabolites. Xenobiotica 1994, 24, 965-976.
- Ashley, D. L.; Bonin, M. A.; Cardinali, F. L.; McCraw, J. M.; Holler, J. S.; Needham, L. L.; Patterson, D. G., Jr. Determining volatile organic compounds in human blood from a large sample population using purge and trap gas chromatography/mass spectrometry. Anal. Chem. 1992, 64, 1021-1029.
- Hill, R. H., Jr.; Shealy, D. B.; Head, S. L.; Williams, C. C.; Bailey, S. L.; Gregg, M.; Baker, S. E.; Needham, L. L. Determination of pesticide metabolites in human urine using an isotope dilution technique and tandem mass spectrometry. J. Anal. Toxicol. 1995, 19, 323-329.
- Yoshida, T.; Andoh, K.; Fukuhara, M. Urinary 2,5-dichlorophenol as a biological index for p-dichlorobenzene exposure in the general population. Arch. Environ. Contam. Toxicol. 2002, 43, 481-485.
- Fourth national report on human exposure to environmental chemicals; Department of Health and Human Services, Centers for Disease Control and Prevention: Washington, DC, 2009; pp 1, 461-463.
- Sexton, K.; Adgate, J. L.; Church, T. R.; Ashley, D. L.; Needham, L. L.; Ramachandran, G.; Fredrickson, A. L.; Ryan, A. D. Children's exposure to volatile organic compounds as determined by longitudinal measurements in blood. Environ. Health Perspect. 2005, 113 (3), 342-349.
- Hill, R. H., Jr.; To, T.; Holler, J. S.; Fast, D. M.; Smith, S. J.; Needham, L. L.; Binder, S. Residues of chlorinated phenols and phenoxy acid herbicides in the urine of Arkansas children. Arch. Environ. Contam. Toxicol. 1989, 18, 469-474.
- Wang, M-J.; Jones, K. C. Behavior and Fate of Chlorobenzenes in Spiked and Sewage Sludge-Amended Soil. Environ. Sci. Technol. 1994, 28 (11), 1843-1852.
- Spain, J. C.; Nishino, S. F., Degradation of 1,4-dichlorobenzene by a Pseudomonas sp. Appl. Environ. Microbiol. 1987, 53 (5), 1010-1019.
- Schwarzenbach, R. P.; Monar-Kubica, E.; Giger, W.; Wakeham, S. G. Distribution, residence time, and fluxes of tetrachloroethylene and 1,4-dichlorobenzene in Lake Zurich, Switzerland. Environ. Sci. Technol. 1979, 13 (11), 1367-1373.
- Howard, P. H. Large Production and Priority Pollutants: 1,4-Dichlorobenzene. Handbook of Environmental Fate and Exposure Data for Organic Chemicals; Lewis Publishers: Chelsea, MI, 1989; Vol. 1, pp 250-262.
- Bouwer, E. J.; McCarty, P. L. Transformations of halogenated organic compounds under denitrification conditions. Appl. Environ. Microbiol. 1983, 45 (4), 1295-1299.
- Bouwer, E. J.; McCarty, P. L. Removal of trace chlorinated organic compounds by activated carbon and fixed-film bacteria. Environ. Sci. Technol. 1982, 16 (12), 836-843.
- Oliver, B. G.; Nicol, K. D. Chlorobenzenes in sediments, water, and selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ. Sci. Technol. 1982, 16 (8), 532-536.
- Schwarzenbach, R. P.; Giger, W.; 56. Hoehn, E.; Schneider, J. K. Behavior of organic compounds during infiltration of river water to ground water. Field studies. Environ. Sci. Technol. 1983, 17, 472-479.
- Young, D. R.; Heesen, T. C. DDT, PCB, and chlorinated benzenes in the marine ecosystem off Southern California. Water Chlorination: Environmental Impact and Health Effects; Jolley, R. L.; Hamilton, D. H.; Gorchev, H., Eds.; Ann Arbor Science: Ann Arbor, MI, 1980; pp 267-290.
- Atkinson, R. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem. Rev. 1985, 85, 69-201.
- Ligocki, M. P.; Leuenberger, C.; Pankow, J. F. Trace organic compounds in rain- II. Gas scavenging of neutral organic compounds. Atmos. Environ. 1985, 19 (10), 1609-1617.
- Wang, M.-J.; Jones, K. C. Uptake of Chlorobenzenes by Carrots from Spiked and Sewage Sludge-Amended Soil. Environ. Sci. Technol. 1994, 28 (7), 1260-1267.
- Zhang, J.; Zhao, W.; Pan, J.; Qiu, L.; Zhu, Y. Tissue-dependent distribution and accumulation of chlorobenzenes by vegetables in urban area. Environ. Int. 2005, 31, 855-860.
- Monferran, M. V.; Wunderlin, D. A.; Nimptsch, J.; Pflugmacher, S. Biotransformation and antioxidant resonse in Ceratophyllum demersum experimentally exposed to 1,2- and 1,4-dichlorobenzene. Chemosphere 2007, 68, 2073-2079.
- Brown, S. K.; Sim, M. R.; Abramson, M. J.; Gray, C. N. Concentrations of Volatile Organic Compounds in Indoor Air: A Review. Indoor Air 1994, 4 (2), 123-134.
- Kostiainen, R. Volatile organic compounds in the indoor air of normal and sick houses. Atmos. Environ. 1995, 29 (6), 693-702.
- Field, R. A.; Phillips, J. L.; Goldstone, M. E.; Lester, J. N.; Perry, R. Indoor/outdoor interactions during an air pollution event in Central London. Environ. Technol. 1992, 13 (4), 391 - 408.
- Shinohara, N.; Ono, K.; Gamo, M. p-Dichlorobenzene emission rates from moth repellents and leakage rates from cloth storage cases. Indoor Air 2008, 18, 63-71.
- De Coensel, N.; Desmet, K.; Sandra, P.; Gorecki, T. Domestic sampling: exposure assessment to moth repellent products using ultrasonic extraction and capillary GC-MS. Chemosphere 2008, 71, 711-716.
- Total Diet Study Market Baskets 1991-3 through 2003-4; U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Food Safety: College Park, MD, 2008; pp i, 51-52.
- Wang, M-J.; Jones, K. C. Occurrence of chlorobenzenes in nine United Kingdom retail vegetables. J. Agric. Food Chem. 1994, 42 (10), 2322-2328.
- Oliver, B. G.; Nicol, K. D. Chlorobenzenes in sediments, water, and selected fish from Lakes Superior, Huron, Erie, and Ontario. Environ. Sci. Technol. 2002, 16 (8), 532-536.
- Pereira, W. E.; Rostad, C. E.; Chiou, C. T.; Brinton, T. I.; Barber, L. B.; Demcheck, D. K.; Demas, C. R. Contamination of estuarine water, biota, and sediment by halogenated organic compounds: a field study. Environ. Sci. Technol. 2002, 22 (7), 772-778.
- Chung, H. Y. Volatile Components in Crabmeats of Charybdis feriatus. J. Agric. Food Chem. 1999, 47 (6), 2280-2287.
- Calamari, D.; Galassi, S.; Setti, F.; Vighi, M. Toxicity of selected chlorobenzenes to aquatic organisms. Chemosphere 1983, 12 (2), 253-262.
- Heitmuller, P. T.; Hollister, T. A.; Parrish, P. R. Acute toxicity of 54 industrial chemicals to sheepshead minnows (Cyprinodon variegatus). Bull. Environ. Contam. Toxicol. 1981, 27 (1), 596-604.
- Buccafusco, R. J.; Ells, 75. S. J.; LeBlanc, G. A. Acute toxicity of priority pollutants to bluegill (Lepomis macrochirus). Bull. Environ. Contam. Toxicol. 1981, 26, 446-452.
- McPherson, C. A.; Tang, A.; Chapman, P. M.; Taylor, L. A.; Gormican, S. J. Toxicity of 1,4-dichlorobenzene in sediments to juvenile polychaete worms. Mar. Pollut. Bull. 2002, 44, 1405-1414.
- Chapman, P. M.; Paine, M. D.; Arthur, A. D.; Taylor, L. A. A triad study of sediment quality associated with a major, relatively untreated marine sewage discharge. Mar. Pollut. Bull. 1996, 32 (1), 47-64.
- Mortimer, M. R.; Connell, D. W., Critical internal and aqueous lethal concentrations of chlorobenzenes with the crab Portunus pelagicus (L). Ecotoxicol. Environ. Saf. 1994, 28 (3), 298-312.
- Mortimer, M. R.; Connell, D. W. Effect of exposure to chlorobenzenes on growth rates of the crab Portunus pelagicus (L). Environ. Sci. Technol. 1995, 29 (8), 1881-1886.
- Wong, P. T. S.; Chau, Y. K.; Rhamey, J. S.; Docker, M. Relationship between water solubility of chlorobenzenes and their effects on a freshwater green alga. Chemosphere 1984, 13 (9), 991-996.
- CDC. NIOSH Pocket Guide to Chemical Hazards: p-Dichlorobenzene; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/niosh/npg/npgd0190.html (accessed April 2010), updated Feb 2009.
- ACGIH. TLVs and BEIs, Based on the Documentation of the Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices; American Conference of Governmental Hygienists Worldwide: Cincinnati, OH, 2008; pp 24, 42.
- Basic Information about p-dichlorobenzene in drinking water; U.S. Environmental Protection Agency, Drinking Water Contaminants. http://www.epa.gov/safewater/contaminants/basicinformation/p-dichlorobenzene.html (accessed April 2010), updated March 2010.

